Our expertise
Our mission is to provide value-added biosimilar products that meet the needs of patients and healthcare professionals. BIOJAMP is committed to simplifying collaboration for you and your patients without compromising the efficacy, quality or safety of the products we offer.

Who we are

BIOJAMP was created through a strategic partnership with Alvotech, a world-class biopharmaceutical company based in Iceland that specializes in the development and manufacture of biosimilar products.
With the launch of the new BIOJAMP division, JAMP Pharma Corporation aims to become your partner of choice in this evolution towards biosimilars and become one of the only companies founded, owned and managed exclusively in Canada, to operate in this growing market.
Making the complex simple
BIOJAMP is committed to simplifying collaboration for you and your patients without compromising the efficacy, quality or safety of the products we offer.
Patient reminder cards:
1310 Nobel Street, Boucherville,
Quebec, QC J4B 5H3, Canada
T 450-449-1236
F 866-399-9091
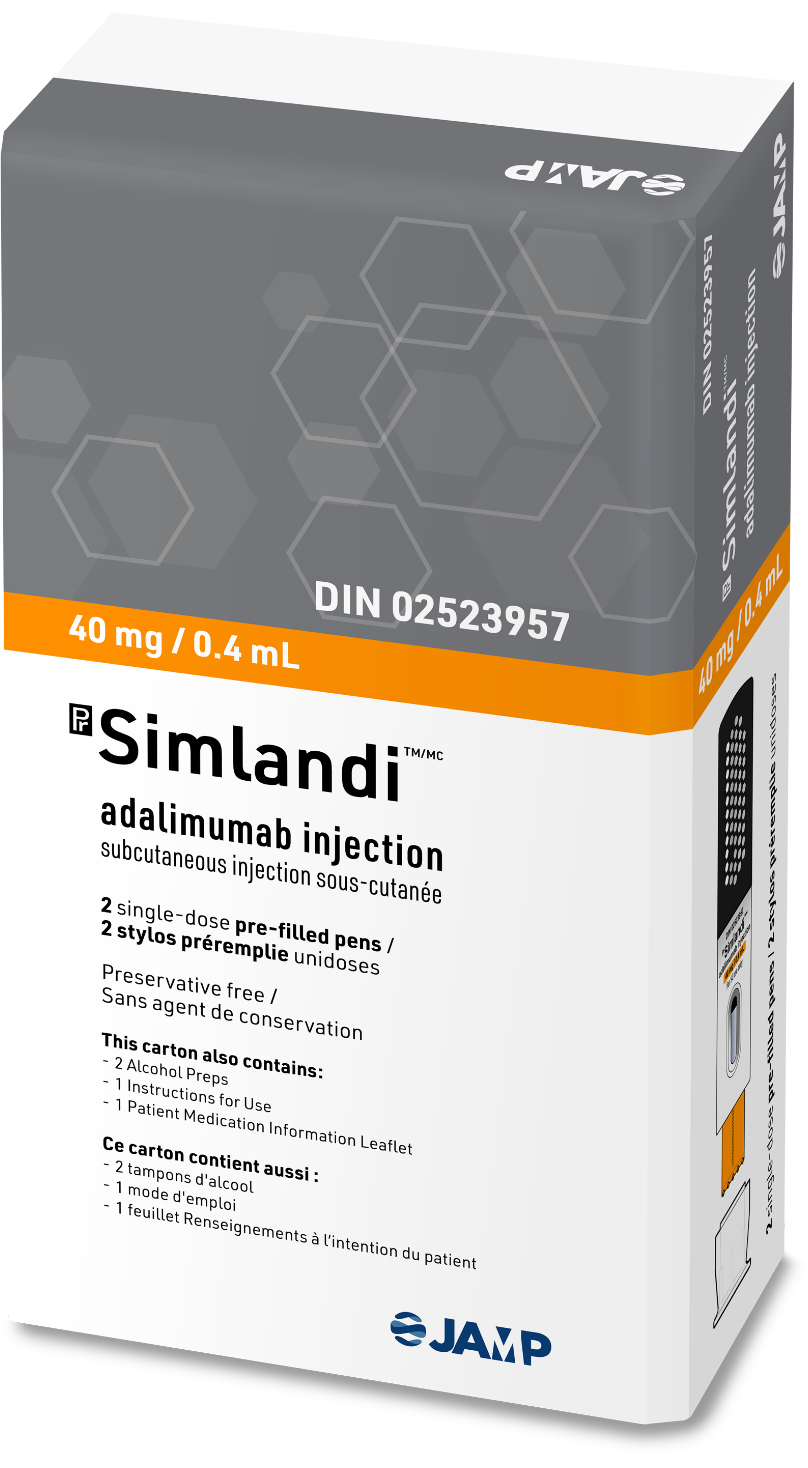
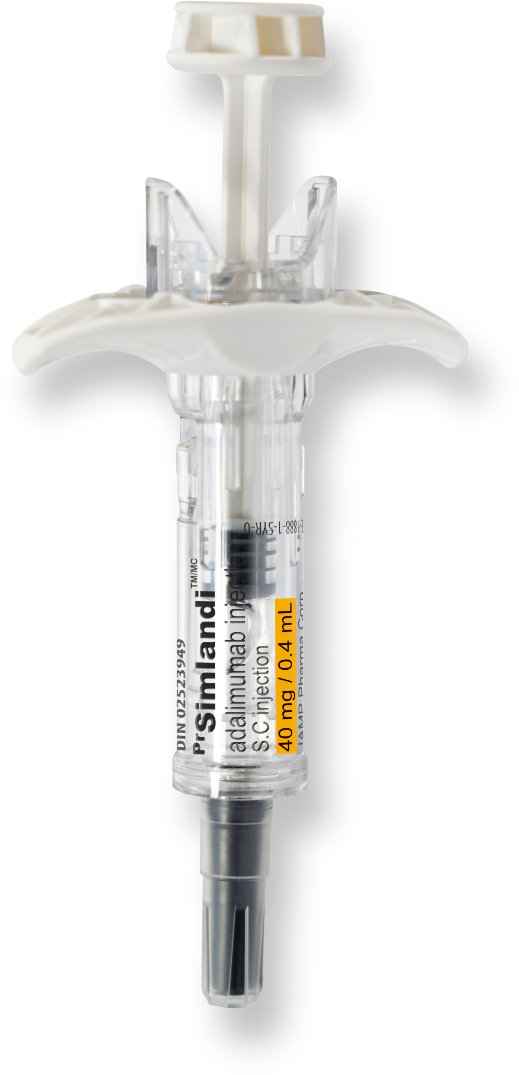
SimlandiTM (adalimumab)
Your patients might be silent about injection site pain. The new high-concentration, low-volume (40 mg/0.4 ml) formula from SimlandiTM (adalimumab) reduces the volume injected by 50%, which could diminish injection site pain1.



Discover YOUR JAMP Care™ Patient Support Program
We are here to help you with your JAMP specialty medications. Through this program, we offer you exclusive services and personalized support to guide you through every step of your patient's treatment.
Simplifying the complex
BIOJAMP is committed to simplifying collaboration for you and your patients without compromising the efficacy, quality or safety of the products we offer.
Patient reminder cards:
1310 Nobel Street, Boucherville,
Quebec, QC J4B 5H3, Canada
T 450-449-1236
F 866-399-9091
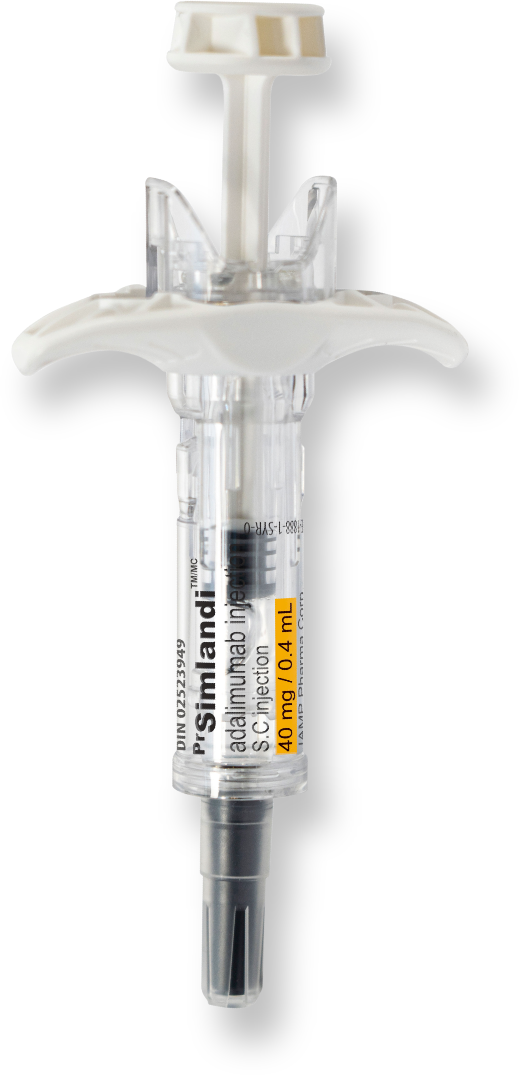

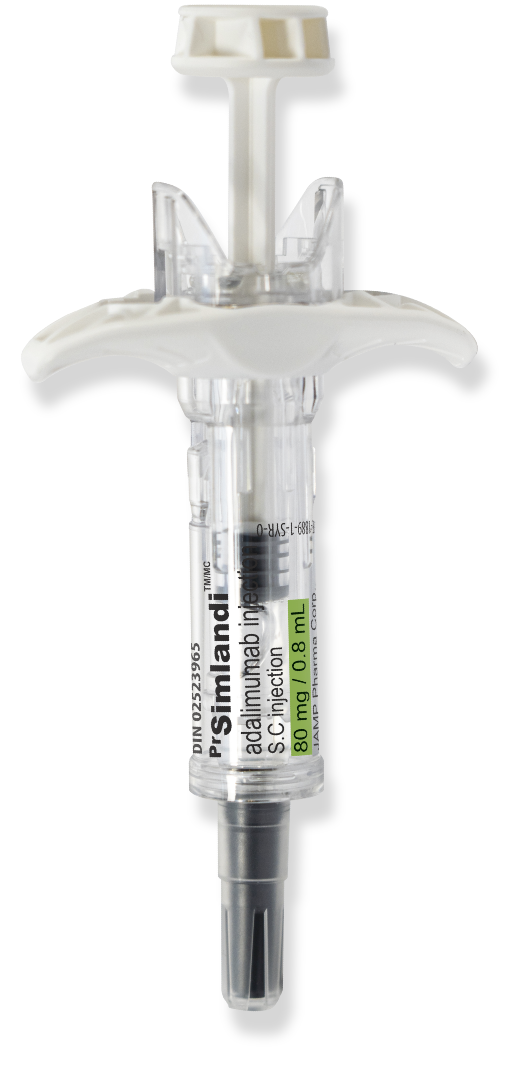
PrSimlandiTM (adalimumab)
Your patients may be silent about the pain they feel on injection.
The new high-concentration, low-volume, (40 mg/0.4 ml) citrate-free formulation of Simlandi™️ (adalimumab) reduces the volume injected by 50%, which could reduce the pain experienced on injection, compared to the current low strength formulation (40 mg/ 0.8 ml)1*
1 In two phase II clinical trials (n = 60 and n = 62) injection site pain felt immediately after administering high-concentration adalimumab (40 mg/0.4 ml) was reduced by a median proportion of 84% in comparison to original adalimumab (40 mg/0.8 ml).
* Nash P, Vanhoof J, Hall S, et al. Randomized Crossover Comparison of Injection Site Pain with 40 mg/0.4 or 0.8 mL Formulations of Adalimumab in Patients with Rheumatoid Arthritis. Rheumatol Ther. 2016;3(2):257-270. doi:10.1007/s40744-016-0041-3
Our goal
To simplify the transition to biosimilars for your patients by offering a range of complementary services and a value-added product, while maintaining its efficacy, safety and immunogenicity.
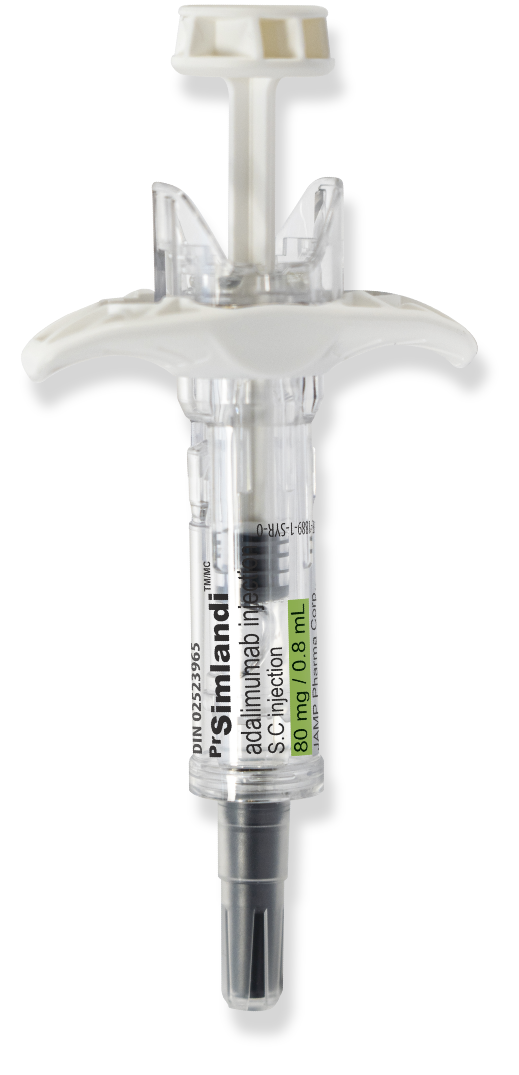
Benefits of Simlandi™ (adalimumab) for your patients:
- The only high concentration, low volume, citrate-free adalimumab distributed by a Canadian company
- Pre-filled syringe with a single 80 mg dose for patients requiring induction doses
- Biosimilar of HUMIRA® high concentration, which is currently not available in Canada
- Ergonomic, two-step autoinjector
- Prefilled syringe with automatically retracting needle for improved injection safety.
- JAMP Care™: YOUR Patient Support Program, offering reliable and efficient services and personalized assistance
Download the PDF: "Indications, clinical use, references"
Download the Product Monograph
Making the complex simple
BIOJAMP is committed to simplifying collaboration for you and your patients without compromising the efficacy, quality or safety of the products we offer.
Patient reminder cards:
1310 Nobel Street, Boucherville,
Quebec, QC J4B 5H3, Canada
T 450-449-1236
F 866-399-9091
Contact
Address
1310 Nobel Street, Boucherville,
Quebec, Canada, J49 5H3
Contact us
T (450) 449-1236
F 866-399-9091
jamppharma.ca
info@biojamp.ca
Simplifying
the complex
Newsletter
Subscribe to our newsletter to receive exclusive information about BIOJAMP.